
Abstract Submission Opens
2025-02-03Abstract Submission deadline
2025-05-05Abstract Author Notification Start
2025-06-05Abstract Author Notification End
2025-07-05Early Registration
2025-07-05Early Registration Deadline
2025-08-05Conference Start
2025-08-26Conference End
2025-08-27Metal-organic frameworks:
Over the past few decades, a large number of synthetic solids have been described that contain metal ions and are linked together by molecular species. This set of compounds has been variously named as metal-organic frameworks of denatured polymers, organic-inorganic hybrid materials, and zeolite-like organic compounds. Metal-organic frameworks, or MOFs for short, are porous and crystalline compounds with strong metal-ligand interactions that were first introduced in 1989 by Hoskin and Robson and then expanded by Yaghi and his colleagues, and the prototypes presented by them had tetrahedral or octahedral structures. Most of the MOFs are in the microporous and porous categories, and only a small portion of them fall into the mesoporous category. MOFs are of interest in chemistry, physics, engineering, and biology. The number of these structures now exceeds 99,000.
The advantages of these structures include the following:
(1) Large opening size, accessibility and adjustability of the pores
(2) Ultra-high porosity (up to 90% free volume)
(3) Large internal surface area, more than 6000 m2/g
(4) The extraordinary diversity of both their organic and inorganic structural components
(5) Post-synthesis structure modifiability for accessibility, reactivity of internal pores, and active sites
These properties have led to the creation of MOFs with potential applications in various fields such as clean energy, mainly as storage for gases such as methane and hydrogen, and as high-capacity adsorbents to meet various separation needs, applications in membranes, catalysts, thin film devices, and bioimaging. In the discussion of catalytic application, some MOFs can form unsaturated metal cation sites by removing the solvent absorbed in their structure. The base metal can then act as a Lewis acid in reactions such as Diels-Alder, Beckmann rearrangements, etc. The high concentration of metal sites in a MAF and their potential accessibility due to the porosity of the framework make MAFs very interesting.
MOFs manufacturing units:
As shown in Figure (1), the metal component in a MOF can be a metal complex or a cluster consisting of two or more metal centers, known as connection points or nodes, and the organic component (ligand) acts as a bridge or connector to connect the metal nodes together in a coordinated manner. To better study and understand these connections and predict the structure during the design of MOFs, the concept of secondary structural unit, which was previously discussed for zeolites, is used. Like zeolite in muffs, the muff structure is formed by the connection of these secondary units together.
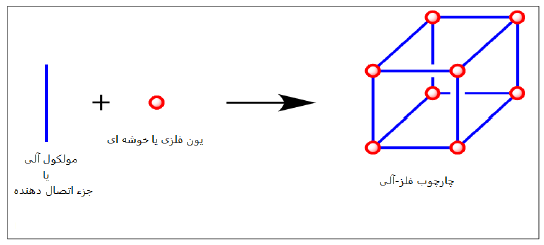
Figure 1 - Outline of the 3D structure of the metal-organic framework
By 2009, 130 secondary structural units were designed and registered, some of which are shown in Figure 2.
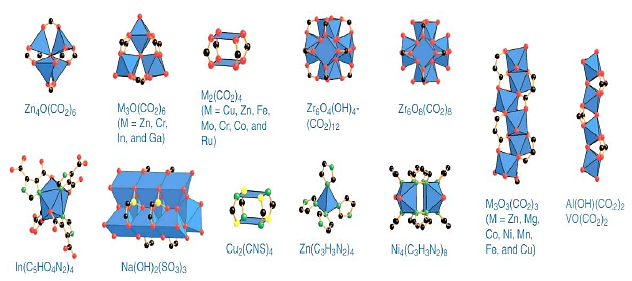
Figure 2. Some secondary structural units of MOFs
Ligands used in metal-organic frameworks include functional groups such as phosphate, sulfonate, iron, carboxylate, or nitrile. Also, several metals are used in the synthesis of MOFs, which are divided into two categories: oxide and nitrate. Among the metals used in the design of MOFs, transition metal ions such as Cr2+, Zn2+, Cu2+, and Fe2+ are the most common. Also, some alkali and alkaline earth metal ions and rare earth metal ions have been used in the structure of MOFs.



















