
Abstract Submission Opens
2025-02-03Abstract Submission deadline
2025-05-05Abstract Author Notification Start
2025-06-05Abstract Author Notification End
2025-07-05Early Registration
2025-07-05Early Registration Deadline
2025-08-05Conference Start
2025-08-26Conference End
2025-08-27COFs
Covalent organic frameworks (COFs) represent another emerging class of nanoporous crystalline materials distinguished from MOFs, which are assembled from organic reactants via covalent bonds
Covalent organic frameworks (COFs) are an emerging class of crystalline polymers with periodic skeletons and ordered pores.
they are one of the most important and dynamic members of the porous organic materials and are constructed using reticular chemistry with the building blocks being connected via covalent bonds.
In this context, the most prominent discovery appeared in 2005 when Yaghi and co-workers developed the crystalline porous organic polymers, i.e., the so-called covalent organic frameworks (COFs) (Fig. 1).
Figure 1. The first COFs synthesized by Yaghi et al..
Different from the synthesis of MOFs usually using organic ligands and metal salts, COFs are generally synthesized via polycondensation reactions of organic monomers (or molecules).
They comprised light elements (such as H, B, C, N, O) and are connected by robust covalent bonds via reticular chemistry (Fig. 2).
Figure 2. The atom, the molecule, and the covalent organic framework.
The noteworthy progress on COFs is mainly due to their selfhealing ability and thermodynamically controlled dynamic covalent chemistry involved in their assembly which results in a long-range ordered crystalline structure. Unlike MOFs, the densities of COFs are usually lower and thus they display excellent stability in organic solvents, and even under varying acidic, basic, oxidative, and reductive conditions. Besides, COFs can withstand harsh conditions and can retain their ordered structure and crystallinity. This is attributed to the metal-free skeletal structure linked via strong covalent bonds rather than coordinate bonds that comprise MOFs.
Based on the covalent connectivity in different dimensions, COFs can be classified into either two-dimensional (2D) laminar structures or three dimensional (3D) extended networks (Fig.3).
Figure 3 - Difference in covalent bonding in two-dimensional and three-dimensional COFs
To date, most efforts in this area have been devoted to 2D COFs, and their construction and characterization have been extensively established. In contrast, 3D COFs have been much less investigated, although their intrinsic porous properties [e.g., remarkably large surface areas (>5000 m2/g), unprecedentedly low densities (∼0.13 g/cm3 ), hierarchical nanopores, and numerous open sites] can make them ideal candidates in diverse research fields, such as molecular adsorption and catalysis
In recent years, COFs have been uniquely designed and synthesized and are explored in numerous applications due to a number of very exclusive advantages, such as low density, pre-designable and well-defined porous structures with uniform pore size ranging from angstroms to nanometers, large surface area, versatile covalent combination of rigid building units having multiconnectivities, structural regularity, tunable and effortless functionalization, sturdy nature that provides resistance to disintegration under adverse conditions and many more. These fascinating advantages have attracted many researchers working in the fields of gas and energy storage, sensing, adsorption of metal ions, catalysis, optoelectrics, and drug delivery, among others
Due to their fascinating properties such as low density, high porosity, and designable functionality, large surface area, structural versatilit, effortless surface modification, and high chemical stability, COFs are being widely deployed in catalytic, sensing, adsorption, gas storage, gas adsorption, energy storage, optoelectronic devices, and many other valuable applications.
Because of their COFs have attracted tremendous interest and exhibited many potential applications in sensing, and separation, catalysisand so forth.
By virtue of the availability of organic units and the diversity of topologies and linkages, COFs have emerged as a new field of organic materials that offer a powerful molecular platform for complex structural design and tailor-made functional development.
A framework, whether a COF or any other extended covalent structure, is composed entirely of two distinct components: the linkers (the building blocks) and the links (the bonds formed between those units after crosslinking). Thus, the organic synthesis of COFs begins with the synthesis of the building blocks and ends with the crosslinking of these building blocks by linking them in a designed manner into a developed framework. The building blocks of COFs typically have a rigid π-column and possess multiple reactive sites. Their solubility in solvent strongly depends on the size of the π-systems and the type of reactive units. Depending on the nature of the chemical reactions, COFs have been synthesized with boroxine, boronate-ester, borosilicate, triazine, imine, hydrazone, borazine, squarine, azine, phenazine, imide, spiroborate, C=C, amide, viologen, urea, and 1,4-dioxine linkages (Fig. 4).
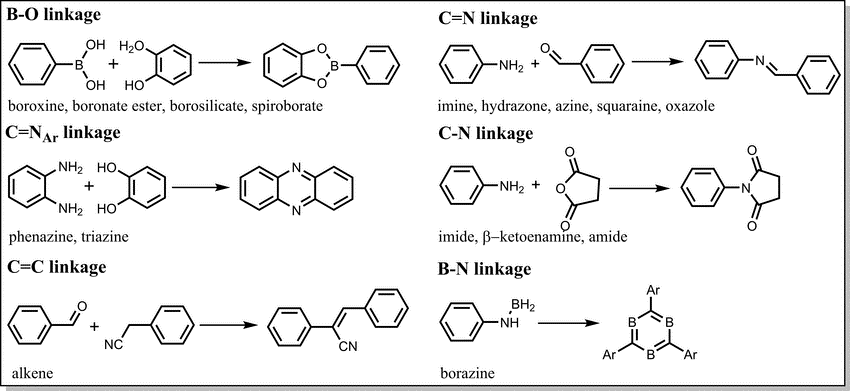
Figure 4. Typical covalent linkages employed in construction of COFs



















